Next |
Prev |
Up |
Top
|
Index |
JOS Index |
JOS Pubs |
JOS Home |
Search
The relative amount of compression/expansion energy that goes into
temperature 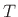 versus pressure
versus pressure  can be characterized by the heat capacity ratio
can be characterized by the heat capacity ratio
where 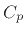 is the specific heat (also called heat
capacity) at constant pressure, while
is the specific heat (also called heat
capacity) at constant pressure, while 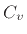 is the specific heat at
constant volume. The specific heat, in turn, is the amount of
heat required to raise the temperature of the gas by one degree. It
is derived in statistical thermodynamics [139]
that, for an ideal gas, we have
is the specific heat at
constant volume. The specific heat, in turn, is the amount of
heat required to raise the temperature of the gas by one degree. It
is derived in statistical thermodynamics [139]
that, for an ideal gas, we have 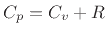 , where
, where  is the ideal
gas constant (introduced in Eq.(B.45)). Thus,
is the ideal
gas constant (introduced in Eq.(B.45)). Thus, 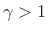 for any
ideal gas. The extra heat absorption that occurs when heating a gas
at constant pressure is associated with the work (§B.2)
performed on the volume boundary (force times distance = pressure times
area times distance) as it expands to keep pressure constant. Heating
a gas at constant volume involves increasing the kinetic energy of the
molecules, while heating a gas at constant pressure involves both that
and pushing the boundary of the volume out. The reason not all
gases have the same
for any
ideal gas. The extra heat absorption that occurs when heating a gas
at constant pressure is associated with the work (§B.2)
performed on the volume boundary (force times distance = pressure times
area times distance) as it expands to keep pressure constant. Heating
a gas at constant volume involves increasing the kinetic energy of the
molecules, while heating a gas at constant pressure involves both that
and pushing the boundary of the volume out. The reason not all
gases have the same  is that they have different
internal degrees of freedom, such as those associated with
spinning and vibrating internally. Each degree of freedom can store
energy.
is that they have different
internal degrees of freedom, such as those associated with
spinning and vibrating internally. Each degree of freedom can store
energy.
In terms of  , we have
, we have
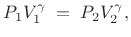 |
(B.46) |
where
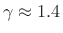 for dry air at normal temperatures. Thus,
if a volume of ideal gas is changed from
for dry air at normal temperatures. Thus,
if a volume of ideal gas is changed from 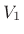 to
to 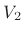 , the pressure
change is given by
, the pressure
change is given by
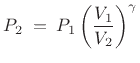 |
(B.47) |
and, converting pressure to temperature via the
ideal gas law
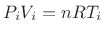 , the temperature change is
, the temperature change is
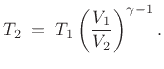 |
(B.48) |
These equations follow from Eq.(B.46) and the ideal gas law
Eq.(B.45).
The value
 is typical for any diatomic
gas.B.31 Monatomic inert gases, on the other hand,
such as Helium, Neon, and Argon, have
is typical for any diatomic
gas.B.31 Monatomic inert gases, on the other hand,
such as Helium, Neon, and Argon, have
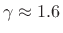 . Carbon
dioxide, which is triatomic, has a heat capacity ratio
. Carbon
dioxide, which is triatomic, has a heat capacity ratio
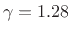 . We see that more complex molecules have lower
. We see that more complex molecules have lower  values because they can store heat in more degrees of freedom.
values because they can store heat in more degrees of freedom.
Next |
Prev |
Up |
Top
|
Index |
JOS Index |
JOS Pubs |
JOS Home |
Search
[How to cite this work] [Order a printed hardcopy] [Comment on this page via email]
![]() versus pressure
versus pressure ![]() can be characterized by the heat capacity ratio
can be characterized by the heat capacity ratio
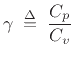
![]() , we have
, we have
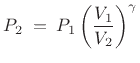
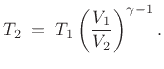
![]() is typical for any diatomic
gas.B.31 Monatomic inert gases, on the other hand,
such as Helium, Neon, and Argon, have
is typical for any diatomic
gas.B.31 Monatomic inert gases, on the other hand,
such as Helium, Neon, and Argon, have
![]() . Carbon
dioxide, which is triatomic, has a heat capacity ratio
. Carbon
dioxide, which is triatomic, has a heat capacity ratio
![]() . We see that more complex molecules have lower
. We see that more complex molecules have lower ![]() values because they can store heat in more degrees of freedom.
values because they can store heat in more degrees of freedom.